Revisiting energy flow in photosynthetic plant cells
29 Jun 2020
By developing innovative methods to visualize energy changes in subcellular compartments in live plants, the team of Dr Boon Leong LIM, Associate Professor of the School of Biological Sciences of The University of Hong Kong, after showing how chloroplasts optimizes its energy efficiency 2 years ago, recently solved a controversial question in photosynthesis: what is the source of NADH (Reduced Nicotinamide adenine dinucleotide) for mitochondria to generate ATP (Adenosine triphosphate)? The results were just published in the journal Nature Communications.
Photosynthesis utilizes light as an energy source for plant chloroplasts to synthesize carbohydrates from water and CO2 molecules. ATPplays an important role in this process, as it promotes plant growth and supply energy for various cellular activities. It had been a general belief that mature plant chloroplasts can import ATP from cytosol since 1969, but it was shown to be untrue by Dr Lim and his team in 2018 (Note 1), through introducing a novel ATP sensor in the subcellular compartments of a C3 model plant, Arabidopsis thaliana. This finding has revised our understanding on chloroplast bioenergetics during daytime and nighttime and how mature chloroplasts optimize energy efficiency.
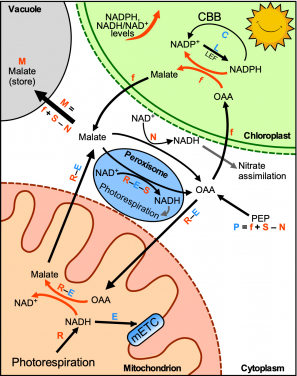
Another unresolved problem in photo-energy is that the source of NADH as a fuel for mitochondria (the major ATP synthesizing organelle in cells) to produce ATP during photosynthesis is unclear. Some researchers suggested that excess reducing equivalents carried by surplus NADPH (Reduced Nicotinamide adenine dinucleotide phosphate) can be exported to the cytosol in the form of malate, which can then enter mitochondria through the malate-OAA shuttle, and converted into OAA and NADH in the mitochondrial matrix.
On the other hand, some researchers proposed that during photorespiration glycine decarboxylase generates a large amount of NADH in mitochondria for ATP production and surplus reducing equivalents carried by NADH is exported by the mitochondrial malate-OAA shuttle to the cytosol.
In the above two pathways, the directions of the malate-OAA shuttle across the mitochondrial membrane during photosynthesis are opposite to each other and therefore this issue had been a matter of debate.
To study this problem, Dr Lim’s group introduced two novel sensors that measure real-time dynamic changes in NADPH levels and NADH/NAD+ ratios (this ratio reflects the reduction/oxidation status of the cellular compartments) in Arabidopsis thaliana. The conventional detection methods require extraction and purification of plant metabolites and determination by chemical methods. These methods have a few drawbacks: in planta measurement and real-time dynamic measurement not feasible; incapable of measurement the energy molecules in different cell types or different subcellular compartments.
“Our novel technique solves all of the problems above. By employing these energy sensors, we found that photorespiration supplies a large amount of NADH to mitochondria during photosynthesis, which exceeds the NADH-dissipating capacity of the mitochondria. Consequently, the surplus NADH must be exported from the mitochondria to the cytosol through the mitochondrial malate-OAA shuttle. (see figure)”, said Ms Sheyli Lim, a PhD student and the first author of a manuscript published in Nature Communications. “Solving this question allows us to understand more about the energy flow between chloroplasts and mitochondria during photosynthesis, which could help us to booth the efficiency of photosynthesis in the future”.
“We are the first group to introduce these three novel energy sensors in plants. They will have wide applications in researches regarding plant bioenergetics. Now we are employing them to study bioenergetics of guard cells, pollen tube growth and C4 plants with international collaborators,” said Dr Lim. “It is a great satisfaction to revisit and clarify some general believes in my field. I wish our findings can eventually help humans to boost agriculture production,’ he added.
Note 1: press release (Oct 2018):
https://www.hku.hk/press/press-releases/detail/c_18582.html
The paper is published in Nature Communications and can be accessed here:
https://www.nature.com/articles/s41467-020-17056-0
Image download:
https://www.scifac.hku.hk/press
For media enquiries, please contact Ms. Cindy Chan, Assistant Director of Communications of HKU Faculty of Science (tel: 3917 5286; email: cindycst@hku.hk) or Dr Boon Leong LIM, Associate Professor of HKU School of Biological Sciences (email: bllim@hku.hk).