Media
HKUMed and JHU teams jointly discover
the role of central nervous system in the control of bone healing
in response to stimulation of metal ions
04 May 2022
A collaborative research project led by Professor Kelvin Yeung Wai-kwok from the Department of Orthopaedics and Traumatology, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong (HKUMed) and Professor Cao Xu from The Johns Hopkins University School of Medicine (JHU) have recently found that the central nervous system (CNS) can effectively modulate bone regeneration when sensory nervous system is being stimulated by metal ions directly injected to the bony tissue, such as magnesium ions (Mg2+), zinc ions (Zn2+), and copper ions (Cu2+). The findings of this study uncover a previously undefined interoceptive circuit in which our CNS can sense and respond to the implanted biomaterials to regulate the process of bone healing. This study has now been published in Nature Communications [link to the publication].
Background
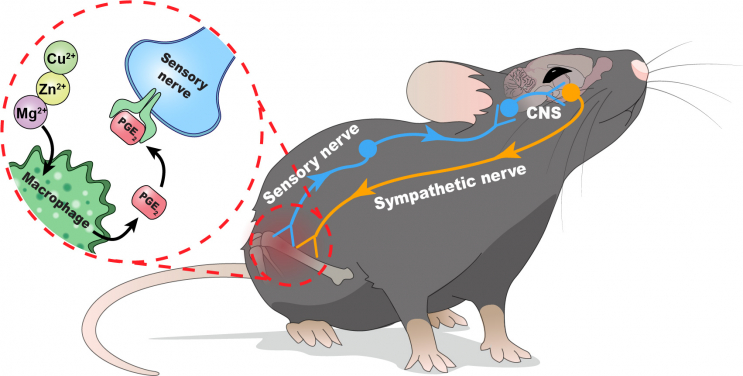
Some metal ions such as Mg2+, Zn2+, and Cu2+, namely divalent cations, have been shown to be promising in bone regeneration. However, their clinical application is still hindered by our limited knowledge of how the molecular and cellular mechanisms of using these divalent metal cations work on bone formation. Recently, the emergence of interoception in neuroscience provides new insight on how CNS controls the internal state of our body. In fact, the skeletal system, the largest organ in human body, has abundant sensory and sympathetic innervations that connect bones with the CNS. The sensory nerve endings extensively extend to bony tissue to recognise and carry signals related to pain, temperature and mechanical stimuli. In addition, bone homeostasis is tightly controlled by several nuclei found in hypothalamus via the regulation of sympathetic nerve activity. However, the exact role of skeletal interoception in bone tissue regeneration driven by metal ions is unknown.
Research methodology and findings
To study the involvement of CNS in metal ion-driven bone healing, the research team injected ion-releasing hydrogel into mouse models with femoral bone defect. The results showed that the inflammatory cytokines secreted from macrophages upon the stimulation of metal ions, particularly prostaglandin E2 (PGE2), serve as inflammatory cues to activate the sensory nerves in bony tissue. When these afferent nerves sense the inflammatory cues in PGE2 receptor 4, they can then convey the interoceptive signals to the CNS. These signals will be processed in the brain and loop back to the injured bone for healing through the sympathetic nervous system. To further verify the findings, the HKUMed and JHU teams tested the hypothesis by using multiple transgenic mouse models, which showed that the early inflammatory response and associated sensation carried by the nervous system are the essential components of the skeletal interoceptive circuit. Moreover, our findings also demonstrated that bone healing induced by metal ions is tightly controlled by the ventromedial hypothalamus located in the CNS through the modulation of sympathetic nerve output.
Significance
The researchers from HKUMed and JHU teams are the first to report the neuromodulatory role of metal ions on new bone formation through skeletal interoception. The discovery of the skeletal interoceptive circuit in bone healing process offers us a new treatment strategy on many challenging cases clinically, such as severe bone fracture, osteoporosis and heterotopic ossification found in some brain injury patients. Compared with the conventional therapeutic agents, the approach of using direct metal ion injection in bone tissue is a promising and cost-effective alternative. The immune-neural axis described in this study can also inspire the development of next-generation of implantable biomaterials that can better harness the healing power of various metal cations.
About the research team
This research was led by Professor Kelvin Yeung Wai-kwok from the Department of Orthopaedics and Traumatology, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong and Professor Cao Xu from Johns Hopkins University School of Medicine. The first author, Dr Qiao Wei, was a post-doctoral fellow under Professor Yeung’s supervision and is now Clinical Assistant Professor at the Faculty of Dentistry, HKU.
Acknowledgements
This study was supported by the Hong Kong Research Grant Council - General Research Fund (#17214516, 17207719) and Hong Kong Innovation Technology Fund (ITS/405/18), the Government of the Hong Kong Special Administrative Region.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).