Media
HKUMed discovers new molecular mechanism
in promoting melanoma angiogenesis and metastasis,
paving the way for new therapeutic opportunities
04 Apr 2022
A research team at LKS Faculty of Medicine, The University of Hong Kong (HKUMed) has discovered a new molecular pathway to govern melanoma angiogenesis and metastasis. The findings provide new insights into how melanoma undergoes metastasis and progression through secretion of pro-angiogenic factor to induce angiogenesis that paves the way for new therapeutic opportunities. The new findings are now published in the leading multidisciplinary science journal, Advanced Science (link to the publication).
Background
Malignant melanoma is a highly aggressive cancer that causes more than 80% of all skin cancer deaths. The high mortality rate of melanoma is primarily attributed to late presentation, drug resistance and metastasis. Cutaneous melanoma is surgically curable in the early stages, with survival rates of up to 98%, but will/may drop to 27% once it undergoes metastasis based on the statistics provided by the Amercian Cancer Society1. This transition involves activation of oncogenes expression to promote secretion of pro-angiogeneic factors for blood vessel recruitment in a process called angiogenesis that increases metastatic propensity. Although current treatments in targeting a well-known pro-angiogenic factor VEGF and its receptor have shown notable antitumour potential, acquired drug resistance compromises their efficacy. In addition, melanoma tumours are highly heterogeneous with subpopulations of cells expressing other pro-angiogenic factors which contribute to melanoma angiogenesis and metastasis, highlighting the importance in identifying new regulators of angiogenesis to provide alternative therapeutic targets for metastatic melanoma.
Research methodology and findings
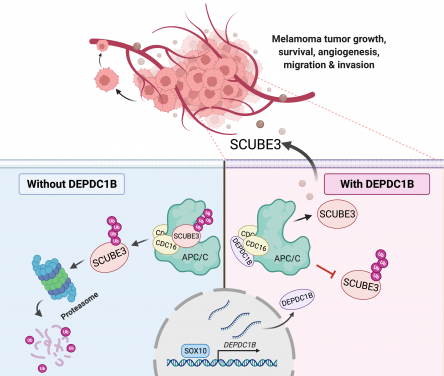
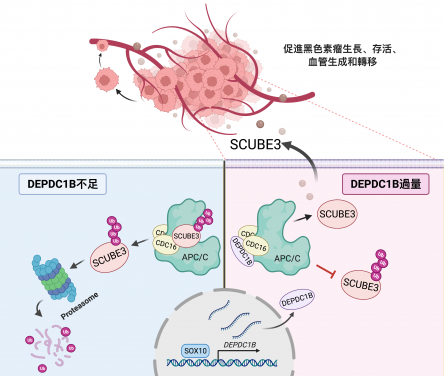
Using melanoma cell lines and the mouse model for in vivo tumour and metastasis assays, the research team found that increased expression of an oncogene DEPDC1B in cutaneous melanoma was significantly associated with poor patient survival and positively correlated with a well-known oncogenic driver SOX10 in melanoma growth and metastasis. Consistently, DEPDC1B functions as a direct downstream target of SOX10 to partly mediate its oncogenic activity. The DEPDC1B-mediated enhancement of melanoma metastatic potential is acquired through secretion of pro-angiogenic factor SCUBE3, which is crucial for promoting angiogenesis. Mechanisitically, DEPDC1B regulates SCUBE3 protein stability through the competitive association with ubiquitin ligase CDC16 to prevent SCUBE3 from undergoing degradation. Importantly, expression of SOX10, DEPDC1B, and SCUBE3 are positively correlated with microvessel density in the advanced stage of melanomas. Altogether, the research findings revealed a new SOX10-DEPDC1B-SCUBE3 regulatory axis that promotes melanoma angiogenesis and facilitates its metastatic progression, suggesting the possibility of targeting secreted SCUBE3 as a therapeutic strategy against metastatic melanoma.
The research team has unravelled a new oncogenic mechanism by which DEPDC1B functions downstream of SOX10 to promote melanoma angiogenesis and metastasis. DEPDC1B competitively interacts with CDC16 in the cytosol to block the ubiquitination and degradation of SCUBE3, enabling stabilised SCUBE3 to be secreted from melanoma cells for blood vessel recruitment to facilitate melanoma growth, survival and metastasis.
Significance of the study
'Our findings underlie the possibility of using both DEPDC1B and SCUBE3 to serve as diagnostic and prognostic markers to improve the clinical assessment of patients with suspected malignant melanomas. Since secreted SCUBE3 is druggable, it is possible to generate SCUBE3 neutralising antibody for the early intervention of melanoma growth, angiogenesis and metastasis. Considering DEPDC1B is oncogenic in other cancer types, our studies have broader implications of DEPDC1B involved in stabilisation of other secreted factors to promote angiogenesis and metastasis in other tumour types,’ said Dr Martin Cheung Chi-hang, Associate Professor, School of Biomedical Sciences, HKUMed, also the lead researcher of the study.
About the research team
The research was led by Dr Martin Cheung Chi-hang, Associate Professor of the School of Biomedical Sciences, HKUMed, as corresponding author. Miss Hu Feng, a final year PhD student, School of Biomedical Sciences was the first author, with assistance from Miss Fong Ki-on, Ms May Cheung Pui-lai, Mr Liang Rui, Miss Li Tsz-wai, and Dr Yang Xintao. Other collaborators included Dr Rakesh Sharma from the Centre for PanorOmic Sciences, HKUMed; Dr Jessica Liu Aijia, Honorary Assistant Professor, Department of Anaesthesiology, School of Clinical Medicine, HKUMed; and Dr Philip Ip Pun-ching, Clinical Associate Professor, Department of Pathology, School of Clinical Medicine, HKUMed.
Acknowledgements
The work was supported by the Hong Kong Research Grants Council - General Research Fund (No.:GRF-17114817) and the Health and Medical Research Fund (HMRF-07183796) of the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region .
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).
1American Cancer Society. Cancer Facts & Figures 2022. Atlanta, Ga: American Cancer Society; 2022.