Media
HKU Virologists Reveal Interplay between CMV and HIV Infection Fosters Research Development and Strategy to Eradicate Latent Viruses
23 Jan 2017
The study was led by led by Professor Chen Zhiwei, Director of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU; and Dr Allen Cheung Ka-loon, Post-doctoral Fellow of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU.
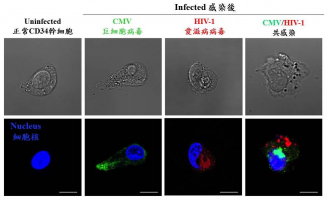
In recent years, breakthroughs in medical treatment of HIV/AIDS greatly extended the survival of infected patients, however, it remains an incurable disease. Previous studies have focused on the depletion of CD4 lymphocytes by human immunodeficiency virus type 1 (HIV-1) infection, but little is known about the infection of CD34 stem cells. The AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU) reveals mechanistic interplay of two viruses, human cytomegalovirus (CMV) and human immunodeficiency virus in co-infecting CD34+ stem cells to establish latent reservoirs. The findings, which show added difficulty in curing HIV-1, have been published recently in Blood Advances of American Society of Hematology.
HIV-1 and CMV
HIV-1 is the causing virus for AIDS. It destroys the white blood cells and impairs the body's immune system, making one susceptible to various opportunistic infections and cancers which rarely affect healthy individuals. As of September 2016, there are 8,243 cumulative reported HIV cases, where more than 80% are men transmitted mainly through sexual contact. The incidence rate has risen from about 300 cases to more than 700 per year in the last ten years.
CMV is a ubiquitous virus that has infected 60-100% of the world’s population. Although it remains asymptomatic in healthy individuals, it poses a fatal threat in congenital infection, organ transplant recipients and AIDS patients.
CMV undergoes life-long latent infection in CD34 stem cells where HIV-1 can also infect them. Past studies have shown that progression to AIDS is faster in those HIV patients already infected with CMV, suggesting that CMV influences HIV-1. But there has been no research to study the interplay of these two viruses in CD34 stem cells.
Research findings
The research team of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU has initiated this study since 2013. They established a cell culture model using and expanding CD34 stem cells from blood, and successfully revealed that HIV-1 and CMV can infect the same cell. These CD34 cells naturally reside in the bone marrow where they can hide from the immune system and antiviral drugs.
In addition, the research team found that pre-existing latent CMV assists HIV-1 infection of CD34 stem cells by “opening a wider door” for the virus. CMV does this by increasing the co-receptors used by HIV-1 to gain entry into the cell as well as decreasing restriction factors. On the other hand, HIV-1 assists CMV by reverting the CD34 stem cell to a more primitive state to achieve a state of quiescent to ensure long-term survival.
Professor Chen Zhiwei, Director of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU says, “The HKU research team is the first group to show in detail how HIV-1 and CMV co-operate in co-infecting CD34+ progenitor cells. The breakthrough findings highlight the importance to understand how both viruses interplay and what we need to consider to develop strategy to eradicate the latent viruses. With recent international focus to purge HIV-1 out of latency in patients, reservoirs of other viruses and niche cell types must be considered.”
About the research team
This research was conducted by the team led by Professor Chen Zhiwei, Director of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU; and Dr Allen Cheung Ka-loon, Post-doctoral Fellow of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU.
Please visit the website at http://www.med.hku.hk/news/ for press photos.
Professor Chen Zhiwei, Director of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU points out that the HKU research team is the first group to show in detail how HIV-1 and CMV co-operate in co-infecting CD34+ progenitor cells. The breakthrough findings have made a step forward to eradicate the latent viruses.