Media
HKUMed designs a novel nanoscale surface structure to accelerate implant-to-bone integration through immune coordination
19 Aug 2021

- - Material surface morphologies of different samples under scanning electron microscope examination, including flat Ti, nano-structure in 90nm, 500nm, 1000nm and 5000nm
- Cellular morphologies of macrophages on different sample surface (scale bars, 10 μm (first column), 5 μm (second column) and 1 μm (third column))
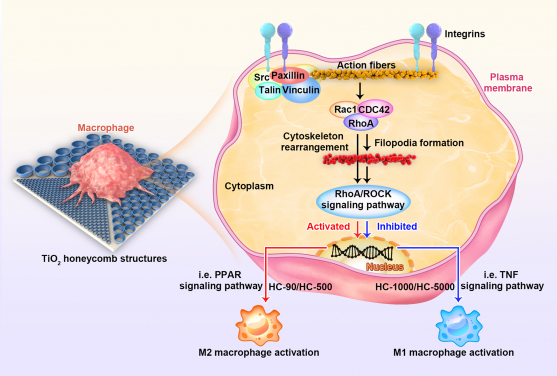
- The cells on 90nm surface extended more “legs” (filopodia) than those on 500nm, 1000nm and 5000nm.
A research team from the Department of Orthopaedics and Traumatology, LKS Faculty of Medicine, The University of Hong Kong (HKUMed) has designed a honeycomb-like nanoscale structure on titanium (Ti) alloy surface to better facilitate implant-to-bone integration. This specific surface structure can substantially accelerate the integration of Ti surface to host bone from 6-8 months to 2-4 months after surgery. This discovery, which provides insight to the design of new generation of prosthesis, bone fracture fixation device and dental implant that require speedy fusion between bone and implant, has been published in Science Advances [link to publication].
Background
Patients with amputation are conventionally managed by prosthetic implantation, using titanium alloys in most cases, with the goal of achieving superior fusion between implant and host bone. However, the fusion often takes 6-8 months to complete. According to medical literature, immune coordination orchestrates the process of bone-to-implant integration by mediating the response of immune cells and the subsequent behaviours of bone cells. Indeed, the immune response induced by the host can be triggered at the implant interface within hours. This inevitable immune response generates an immune tissue microenvironment adjacent to the implanted biomaterials that determines the fate of bone-to-implant integration.
Emerging evidence indicates that topographical cues on the implant material surface are essential to facilitate the integration between bone and implant when the immune microenvironment in bone tissue is properly modulated. However, the underlying mechanism of how surface topographical cues modulate immune cell behaviours remains unknown.
About the study
The HKUMed research team therefore designed a series of honeycomb-like nano-structures on titanium surface ranging from nanometer to micrometer scale to mimic various spatial confinements to the immune cells. We found that macrophages (a kind of non-activated immune cells) can be divided into different subsets according to the surface topographical cues provided by various nano-structures.
As the study reveals, the honeycomb-shaped nano-structures, particularly those in 90 nm, can signal M0 macrophages to switch into anti-flammatory subset (M2) via a specific signalling pathway known as RhoA/ROCK. By providing limited space to the cells that facilitates the anchorage of macrophages substantially, the nano-structure upregulates three canonical guanosine triphosphatases (RhoA, Rac1, and CDC42) belonging to Rho family and triggers M2 macrophage polarisation via RhoA/ROCK. This tailored immune tissue microenvironment favours mesenchymal stem cells towards osteogenesis, thus resulting in superior bone-to-implant integration.
‘The nano-structures can be applied on patients who need prosthesis implantation, bone fracture fixation and even dental implants, reducing up to two-thirds of the implant-to-bone integration time. They can also be used in other bone implant materials such as cobalt-chromium alloy for total joint replacement,’ said Professor Kelvin Yeung Wai-kwok of Department of Orthopaedics and Traumatology, HKUMed, who led the research. ‘Subject to the results of further research and clinical trials, we may also use the honeycomb structures to house medication for pain relief and antibacterial treatment in the future.’
About the research team
This research was led by Professor Kelvin Yeung Wai-kwok of Department of Orthopaedics and Traumatology, HKUMed. The first author Zhou Yizhou is a PhD student of Department of Orthopaedics and Traumatology, HKUMed. Dr Liu Xiangmei from Hubei University, who was a post-doctoral fellow at HKUMed under Professor Yeung’s supervision, and Professor Yang Cao from Huazhong University of Science and Technology are the co-corresponding authors. The research interests of Professor Yeung’s team include orthopaedic biomaterials, musculoskeletal tissue regeneration and anti-bacterial infection.
Acknowledgments
This work is jointly supported by National Key R&D Program of China (2018YFA0703100), General Research Fund of Hong Kong Research Grant Council (nos. 17207719 and 17214516), Hong Kong Health and Medical Research Fund (no.19180712), the National Science Fund for Distinguished Youth Scholar (no. 51925104), Shenzhen Science and Technology Funding (JSGG20180507183242702), HKU-SZH Fund for Shenzhen Key Medical Discipline (SZXK2020084), and Sanming Project of Medicine in Shenzhen “Team of Excellence in Spinal Deformities and Spinal Degeneration” (SZSM201612055).
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).