Media
HKUMed reports that timely booster vaccination reduces Omicron breakthrough infections and COVID-19 severity
04 Jan 2023
Researchers at the AIDS Institute, Department of Microbiology, School of Clinical Medicine, and the State Key Laboratory of Emerging Infectious Diseases, LKS Faculty of Medicine of The University of Hong Kong (HKUMed) reveals that the timely third vaccination of either CoronaVac or BNT162b2 is critical to induce activated virus-specific memory B cells[1] and Omicron cross-reactive T cell responses, leading to significantly reduced frequencies of breakthrough infection and disease severity. Neutralising antibody potency against BA.2.12.1 and BA.4/5 was enhanced by the BA.2 breakthrough infection and bivalent booster vaccination among people who had received prior 3 doses of vaccines. The research paper is now online in the journal of The Lancet Regional Health – Western Pacific [link to the publication].
Background
The explosive spread of SARS-CoV-2 Omicron variants since the end of 2021 in Hong Kong, the city of universal masking in the world, has resulted in the fifth and the largest wave of epidemic with more than 2 million infections and over 10,000 deaths.[2] Omicron BA.2 dominated this fifth wave due to its high transmissibility and immune evasion, leading to the mortality of 14.39%[3] among the unvaccinated elderly aged 80 and above. The majority of pre-vaccinated people, however, experienced mild clinical symptoms after the BA.2 breakthrough infection. Since the immunogenicity of CoronaVac (CorV) is weaker than that of BNT162b2 (BNT), it was essential to determine how vaccine-induced immune responses protected Omicron BA.2 breakthrough infections. Moreover, with subsequent introduction of BA.4/5 and other variants into the region, immune responses boosted by BA.2 breakthrough infection should be tested for cross-reactive neutralising antibodies against BA.4/5 and other variants.
Research methods and findings
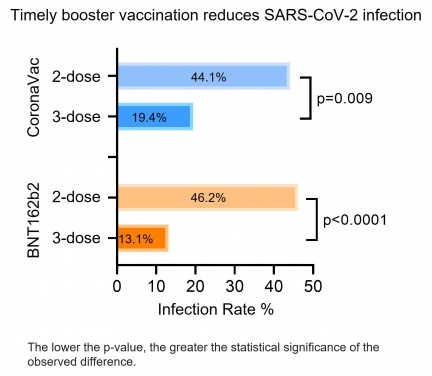
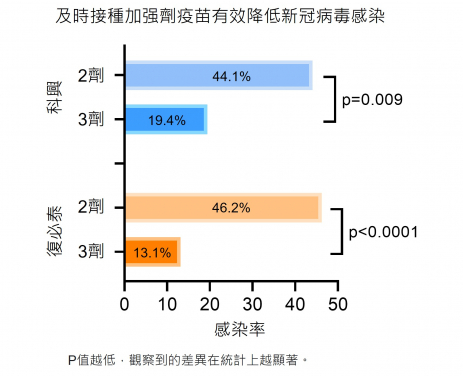
Researchers investigated the vaccine efficacy against the Omicron BA.2 breakthrough infection among 470 local public servants who had received at least two doses of either BNT or CorV vaccines before BA.2 arrived Hong Kong. Antibody and cellular immune responses after three-dose vaccination were characterised and correlated with clinical characteristics of BA.2 infection. The research team found that the timely third-dose vaccination provided better protection with lower incidence rates of breakthrough BA.2 infections (2×BNT 46.2% vs 3×BNT 13.1%, p<0.0001; 2×CorV 44.1% vs 3×CorV 19.4%, p=0.003).
Investigation of immune responses in three-dose vaccination cohorts revealed that the third-dose vaccination-activated spike (S)-specific memory B cells and Omicron cross-reactive T cell responses correlated with reduced frequencies of breakthrough infections and disease severity rather than with types of vaccines. Moreover, the frequency of S-specific activated memory B cells was significantly lower in infected vaccinees than uninfected vaccinees before vaccine-breakthrough infection whereas the amount of S-specific CD4 T cells were negatively associated with viral clearance time. Critically, BA.2 breakthrough infection boosted cross-reactive memory B cells with enhanced cross-neutralising antibodies to Omicron sub-lineages, including BA.2.12.1 and BA.4/5, in all vaccinees tested.
Significance of the study
‘Our findings highlight the importance of activated memory B cells and Omicron cross-reactive T cell responses for prevention and disease protection against Omicron BA.2. Since BA.2 breakthrough infections mainly recalled vaccine-induced antibody against ancestral strain, emphasise the importance of monitoring cross protection against newly emerged viral variants induced by breakthrough infection and the new bivalent COVID-19 vaccine,’ said Dr Zhou Runhong, Research Assistant Professor, Department of Microbiology, School of Clinical Medicine, HKUMed.
‘It is encouraging to see that CoronaVac-induced memory B and T cells were recalled quickly for protection against the Omicron breakthrough infection,’ remarked by Professor Chen Zhiwei, Director of AIDS Institute and Professor of the Department of Microbiology, School of Clinical Medicine, HKUMed, who led the study. ‘We, however, should strengthen public surveillance on highly pathogenic viral escape variants to prevent their attack to vaccinated people.’
About the research team
The research is led by Professor Chen Zhiwei, Director of AIDS Institute and Professor of the Department of Microbiology, School of Clinical Medicine, HKUMed; and was conducted primarily by Dr Zhou Runhong, Research Assistant Professor; Liu Na, PhD candidate; Dr Li Xin, Clinical Assistant Professor at the Department of Microbiology, School of Clinical Medicine, HKUMed, shared the first authorship. This collaborative team also includes Peng Qiaoli, Kwok Hau-yee; Huang Haode, Yang Dawei and Du Zhenglong and Au Ka-kit, Research Assistants; Yiu Cheuk-kwan from Queen Mary Hospital; Cai Jianpiao, Assistant Research Officer, Department of Microbiology, School of Clinical Medicine, HKUMed; Dr Kelvin To Kai-wang, Clinical Associate Professor, Department of Microbiology, School of Clinical Medicine, HKUMed; Professor Ivan Hung Fan-ngai, Department of Medicine, School of Clinical Medicine, HKUMed; Professor Yuen Kwok-yung, Henry Fok Professor in Infectious Diseases and Chair of Infectious Diseases, Department of Microbiology, School of Clinical Medicine, HKUMed; Academician, Chinese Academy of Engineering; Director of the State Key Laboratory of Emerging Infectious Diseases, The University of Hong Kong.
Acknowledgements
This study was supported by the Hong Kong Research Grants Council Collaborative Research Fund (C7156‐20GF, C1134‐20GF and C5110‐20GF) and Health and Medical Research Fund (19181012); Shenzhen Science and Technology Program (JSGG20200225151410198 and JCYJ20210324131610027); the Hong Kong Health@InnoHK, Innovation and Technology Commission; and the China National Program on Key Research Project (2020YFC0860600, 2020YFA0707500 and 2020YFA0707504); and donations from the Friends of Hope Education Fund. Professor Chen Zhiwei’s team was also partly supported by the Hong Kong Theme‐based Research Scheme (T11‐706/18‐N and T11-709/21-N and Wellcome Trust (P86433).
About the Department of Microbiology, School of Clinical Medicine, HKUMed
The academic staff of Department of Microbiology are actively involved in clinical service and basic research. Postgraduate students may pursue studies on various aspects of microbiology and infectious diseases leading to an MPhil or PhD degree. The Master of Medical Sciences programme offers an opportunity to postgraduates interested in more in-depth studies on the biomedical aspects of clinical microbiology and infectious disease. In addition, the clinical staff of the Department also participate in the training of clinical microbiologists in Hong Kong and Shenzhen. The Infectious Disease Courses and Postgraduate Diploma Programme provides a unique avenue for the training of qualified medical practitioners in infectious diseases.
To promote knowledge exchange, research activities at the Department of Microbiology, School of Clinical Medicine, HKUMed can be viewed through www.microbiology.hku.hk.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).
[1] Memory B cell: an immune cell type which can be trained by vaccine and viral infection. They memorise the characteristics of the antigen that activated their parent B cell during initial priming. Once they encounter the same antigen, antibody response will be recalled for viral elimination.
[2] https://www.chp.gov.hk/files/pdf/local_situation_covid19_en.pdf
[3] https://www.covidvaccine.gov.hk/pdf/death_analysis.pdf