Media
HKUMed achieves breakthrough in photoactivatable nanomedicine
for the treatment of age-related macular degeneration
23 Nov 2023
A research team from HKUMed developed a photoactivatable prodrug nanomedicine for age-related macular degeneration (AMD) therapy. The research team members include: (from left) Dr Li Jia, Xu Shuting, Dr Wang Weiping, Dr Fan Ni, and Long Kaiqi.
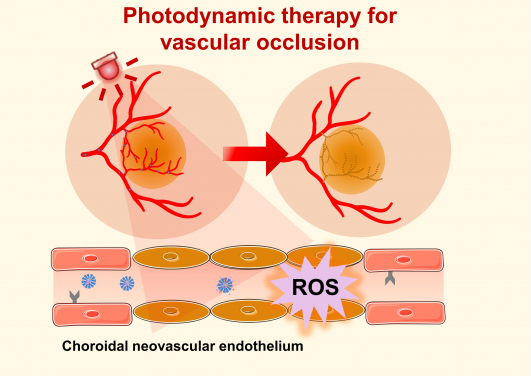
Photodynamic therapy (PDT) offers a clinical solution by utilising non-toxic photosensitisers activated by specific wavelengths of light to generate reactive oxygen species (ROS), which can damage and obliterate neovascularisation.
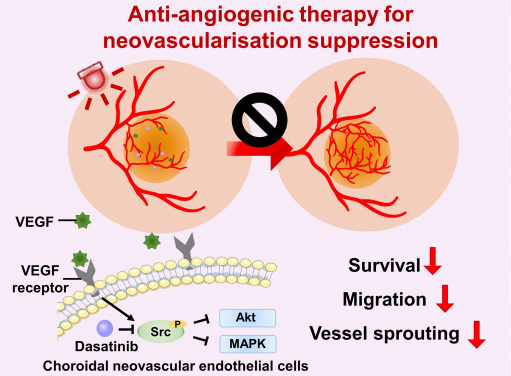
Anti-angiogenic therapy suppresses choroidal neovascularisation (CNV) by inhibiting vascular endothelial growth factor (VEGF)-related pathways. The study represents the first attempt at integrating a photoactivatable anti-angiogenic agent with a photosensitiser into a single nanoformulation for age-related macular degeneration treatment, which opens up new avenues for the development of minimally-invasive therapeutics for AMD and other neovascular ocular disorders.
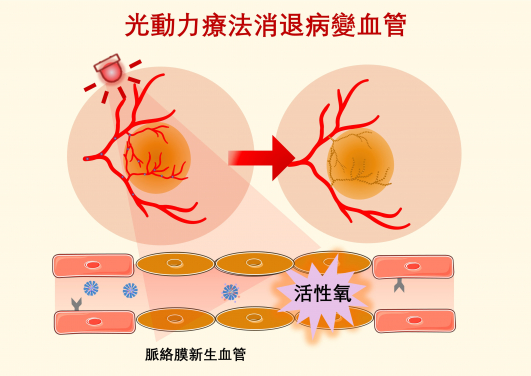
Photodynamic therapy (PDT) offers a clinical solution by utilising non-toxic photosensitisers activated by specific wavelengths of light to generate reactive oxygen species (ROS), which can damage and obliterate neovascularisation.
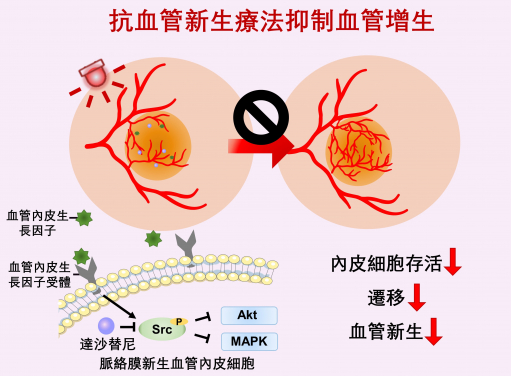
Anti-angiogenic therapy suppresses choroidal neovascularisation (CNV) by inhibiting vascular endothelial growth factor (VEGF)-related pathways. The study represents the first attempt at integrating a photoactivatable anti-angiogenic agent with a photosensitiser into a single nanoformulation for age-related macular degeneration treatment, which opens up new avenues for the development of minimally-invasive therapeutics for AMD and other neovascular ocular disorders.
Researchers at the LKS Faculty of Medicine of the University of Hong Kong (HKUMed), and collaborators from the Zhongshan Ophthalmic Centre of Sun Yat-sen University, Guangzhou, have developed a light-activatable prodrug nanomedicine for age-related macular degeneration (AMD) therapy. Through the intravenous injection of the nanomedicine and application of light irradiation to diseased eyes, anti-angiogenic and photodynamic combination therapy can be activated, offering a minimally invasive alternative for the treatment of AMD and other ocular disorders characterised by abnormal blood vessel growth. The research has been published in Advanced Science [link to publication], and a Patent Cooperation Treaty application was filed based on the research.
Background
According to the Hong Kong Eye Survey data published in 2019, the prevalence of early AMD among individuals aged 70 and older has reached 7.5% in Hong Kong1, making AMD the second most common cause of visual impairment and blindness in the adult population of Hong Kong. Currently, intravitreal injections of antibodies against vascular endothelial growth factor are the first-line treatment for wet AMD. However, this invasive procedure is uncomfortable for patients, and carries the risk of serious ocular complications, such as endophthalmitis and retinal detachment. Therefore, there is an urgent need for novel formulations that enable the delivery of anti-angiogenic agents into the eye without an intravitreal injection, such as intravenous injection.
Additionally, anti-angiogenic agents have limited efficacy in regressing existing neovascularisation. Photodynamic therapy (PDT) offers a clinical solution by utilising non-toxic photosensitisers activated by specific wavelengths of light to generate reactive oxygen species (ROS), which can damage and obliterate neovascularisation. PDT is widely used for the treatment of polypoidal choroidal vasculopathy, a common subtype of wet AMD in Asia. Therefore, combining anti-angiogenesis therapy with PDT may offer a more effective approach to treating wet AMD, thus helping slow down the progression of the disease and improve the vision outcome for patients.
Research methods and findings
The research team designed a novel photoactivatable prodrug nanosystem. After a single intravenous injection of the nanoparticles into a choroidal neovascularisation mouse model, red-light irradiation of the mouse’s eye activated the nanoparticles to generate ROS, which not only causes the regression of abnormal neovascularisation, but also triggers the release of anti-angiogenic drugs from the nanoparticles to inhibit the growth of new blood vessels. This combinational therapy demonstrated excellent therapeutic efficacy without any noticeable systemic or ocular side effects.
Research significance
The study represents the first attempt at integrating a photoactivatable anti-angiogenic agent with a photosensitiser into a single nanoformulation for AMD treatment. The treatment procedure is simple and safe, as the therapeutic effect of the anti-angiogenic agent and photosensitiser in ocular lesions can be achieved through the intravenous administration of nanoparticles and light irradiation to the eye. This pioneering research may open up new avenues for the development of minimally-invasive therapeutics for AMD and other neovascular ocular disorders. The formulation uses US FDA-approved therapeutic agents and excipients, making it promising for future clinical translation.
About the research team
The study was led by Dr Wang Weiping, Associate Professor of Dr Li Dak-Sum Research Centre and Department of Pharmacology and Pharmacy, HKUMed, and Principal Investigator of the State Key Laboratory of Pharmaceutical Biotechnology, HKU. The other corresponding author was Professor Liang Xiaoling, Department of Ophthalmology, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, State Key Laboratory of Ophthalmology, Sun Yat-Sen University, and Zhongshan Ophthalmic Centre, Sun Yat-sen University; the co-first authors were Xu Shuting, PhD candidate, Department of Pharmacology and Pharmacy, HKUMed, and Dr Cui Kaixuan, Postdoctoral fellow, Department of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-Sen University. Other researchers included Long Kaiqi, PhD candidate, Department of Pharmacology and Pharmacy, HKUMed; Dr Li Jia and Dr Fan Ni, Postdoctoral fellows, Department of Pharmacology and Pharmacy; and Professor Lam Wai-ching, Head and Clinical Professor of the Department of Ophthalmology, Vancouver General Hospital and University of British Columbia, Canada, and Honorary Clinical Professor, Department of Ophthalmology, School of Clinical Medicine, HKUMed.
Acknowledgements
This work was supported by the National Natural Science Foundation of China Excellent Young Scientists Fund (No. 82222903) and the National Natural Science Foundation of China (No. 82271099).
Media enquiries
Please contact LKS Faculty of Medicine of the University of Hong Kong by email (medmedia@hku.hk).
1 https://iovs.arvojournals.org/article.aspx?articleid=2743658
