Media
HKU AIDS Research Institute Discovers a New Immune Pathway
Critical to Treatment of Gut Inflammation in HIV-1 Infection
24 Aug 2017

Professor Chen Zhiwei, Director of the AIDS Institute and Professor of Department of Microbiology (Right), and Dr Allen Cheung Ka Loon, Postdoctoral Fellow of Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU took a group photo at the press conference.
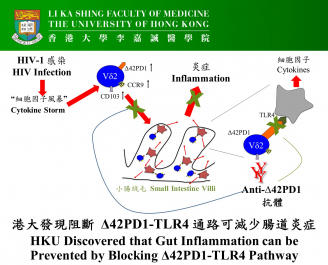
AIDS is commonly called "an incurable disease of the century". To date, the pathogenesis of this sexually transmitted disease remains incompletely understood and there is still no cure for AIDS. The research team of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU) made a breakthrough discovery on a novel Δ42PD1-TLR4 immune pathway that likely accounts for acute gut inflammation in HIV-1 patients. More importantly, the team generated an antibody against this inflammatory pathway and tested it in a humanised mice model to successfully block HIV-1-induced inflammation in small intestines. Their study not only solves the mystery of an inflammatory mechanism during acute HIV-1 infection, but also anticipates to develop the antibody as a new immunotherapy to fight against AIDS and other inflammation-related diseases. The new findings of this research are recently published in the international prestigious journal, Nature Microbiology.
AIDS and gut inflammation
AIDS stands for Acquired Immune Deficiency Syndrome. It is primarily caused by a virus called Human Immunodeficiency Virus Type One (HIV-1). The virus mainly destroys the white blood cells called CD4 T lymphocytes and weakens the body’s immune system. As a result, the body cannot fight off opportunistic infections and cancers, and therefore progress to AIDS. In Hong Kong, about 80% of HIV positive people were infected through unprotected sexual contacts. As of March 2017, there are 8,612 cumulative reported HIV cases in Hong Kong, where the incidence rate does not seem to be slowing down. Of these, more than 80% are men transmitted mainly through homosexual contact.
The initial and massive depletion of CD4 T cells occurs in the intestines within 2-3 weeks after HIV-1 infection, largely due to inflammation-associated immune activation in close proximity with the primary site of infection in the vagina or colon, together with inflammation of the gut. The consequences are the breakdown of the gut barrier and allowing microbes to breach into the inner layers of the intestines. However, the mechanism underlying the initiate intestinal inflammation is not fully defined. An effective vaccine for human use still requires much effort, it is therefore important to identify new preventive strategies against HIV-1 pathogenesis.
Research findings
This study was initiated by the research team of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU since 2013 when they discovered the new molecule named as Δ42PD1 (published in Molecular Therapy). Their research showed that Δ42PD1 is primarily found on the gut-homing γδ subset of T cells that is highly induced following acute HIV-1 infection in patients and correlated with inflammatory cytokines.
Using a humanised mouse model, the team found that Δ42PD1+γδ-T cells could migrate to the small intestines and cause inflammation through TLR4. Their research did not stop here, the team generated an antibody that not only blocks the function of the Δ42PD1/TLR4 pathway but also reduces HIV-1 manifestations in the gut when injected at least 2 hours before the arrival of the Δ42PD1+γδ-T cells in the gut. As a result, inflammation is markedly reduced in the small intestines where the epithelial layer remains intact, thus protecting against microbial translocation. The research team hopes to further extend their research to other mucosa-associated inflammatory diseases, such as, influenza infection, colitis and cancer, etc.
Dr Allen Cheung Ka Loon, Postdoctoral Fellow of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU says, “By discovering a new pathway that could promote HIV-1 pathogenesis in the gut, we understood better what is happening in acute HIV-1. Having also found a way to block it, we can provide new measures in relieving the initial pathological inflammatory response and clinical burden in chronic HIV-1 patients.”
Professor Chen Zhiwei, Director of the AIDS Institute and Professor of Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU says, “The HKU research team, for the first time, discovers a new immune pathway that accounts for gut-associated inflammation. Our findings also demonstrated that by blocking this pathway it is possible to prevent HIV-1-induced mucosal damages. We believe this pathway is important for other inflammatory diseases not limited to HIV-1, and hope to develop the antibody as a new immunotherapy to benefit patients quickly.”
About the research team
This research was performed in collaboration with the City University of Hong Kong, Shenzhen Third People’s Hospital, Beijing You-An Hospital and First Affiliated Hospital of China Medical University, Shenyang.
The research was conducted by the team led by Professor Chen Zhiwei, Director of the AIDS Institute and Professor of Department of Microbiology, and Dr Allen Cheung Ka Loon, Postdoctoral Fellow of Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU.
Media enquiries
Please contact Li Ka Shing Faculty of Medicine of The University of Hong Kong by email (medkefa@hku.hk).
Professor Chen Zhiwei, Director of the AIDS Institute and Professor of Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU points out that the HKU research team, for the first time, discovers a new immune pathway that accounts for gut-associated inflammation. The findings also demonstrate that by blocking this pathway it is possible to prevent HIV-1-induced mucosal damages.
Dr Allen Cheung Ka Loon, Postdoctoral Fellow of the AIDS Institute, Department of Microbiology, Li Ka Shing Faculty of Medicine, HKU says that by discovering a new pathway that can promote HIV-1 pathogenesis in the gut as well as the way to block it, the new measures are expected to relieve the initial pathological inflammatory response and clinical burden in chronic HIV-1 patients.