Media
HKUMed identifies a new mechanism to promote bone health by stimulating immune system through the influx of magnesium ions
07 Sep 2021
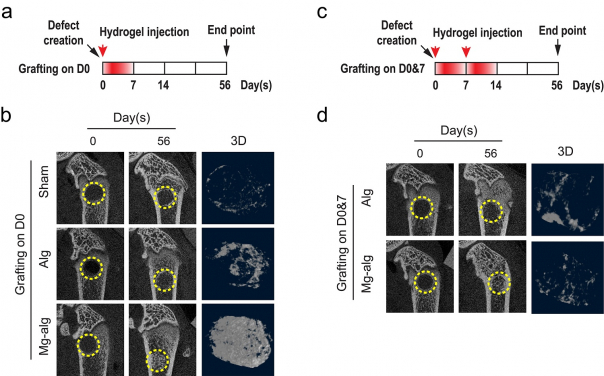
The effect of different Mg2+ delivery strategy on the bone regeneration of rat femoral defect. a) Mg2+-crosslinked alginate hydrogel was injected into the femoral defect in rats right after the injury, hence the release of Mg2+ was limited to the first week after the injury; b) micro-CT images and reconstructed 3D images of the defects in rat femora without grafting, grafted with pure alginate or Mg2+ releasing alginate on day 56; c) Mg2+-crosslinked alginate was injected into the femur defect in rats immediately and on the seventh day after the injury to allow sustained delivery of Mg2+ in the first two weeks of injury; d) the corresponding micro-CT and reconstructed 3D images of the defects in rat femur on day 56 when the grafting was repeated.
A team of researchers from the LKS Faculty of Medicine, The University of Hong Kong (HKUMed) has identified a new mechanism to stimulate bone growth through the modulation of macrophage functions using the influx of magnesium ion (Mg2+). The discovery provides a new insight of how minerals such as Mg2+ play a role in bone healing, which may inspire the development of new strategies for bone fracture treatment or even osteoporosis management. The discovery has been published in Nature Communications [link to the publication].
Background
Bone tissue has a substantial capacity to repair itself after injury. However, the natural healing of bone takes time to complete and it often fails to restore to its original strength and structure. As one of the key trace elements in bone tissue, magnesium is reported in a lot of literature to be essential in bone homeostasis and metabolism and Mg2+ containing biomaterials have shown a superior bone-inducing capacity. Despite the widespread observations of Mg2+ in bone health, the definite role of Mg2+ in bone healing has not been systematically studied, since bone regeneration involves a complex cellular cascade spanning from early tissue inflammation to later bone formation and remodelling. The orthopaedic implant made of degradable magnesium has also been considered to replace the conventional non-degradable titanium implant in clinical practice, because some researchers have found that the derivatives of magnesium may stimulate regeneration of bone tissue. However, the detrimental effects of excessive release of Mg2+ have also been documented. The dichotomised results may be attributed to the partial understanding of the multifaceted roles of Mg2+ in bone healing involved in the complex biological system, thus warranting further research.
In addition, the monocyte-macrophage cell lineage has been recognised as a key player in acute inflammation response to biomaterial implants and later bone regeneration, as the cells can respond to environmental cues that may regulate the bone homeostasis within tissue microenvironment. The research team has thus set up an array of simulated scenarios of immune inflammation triggered by bone injury and introduced Mg2+ at different concentration levels in the therapy. It was discovered that the speed and quality of bone regeneration can be manipulated by modulating the concentration level of Mg2+ used in various stages of bone recovery.
Research methodology and findings
To unveil the underlying mechanism, the research team has therefore designed a few of clinically relevant rat models with femoral bone defect in this study. In brief, the team had delivered Mg2+ containing sodium alginate hydrogels to the bone defect at different stages of healing. The results suggested that Mg2+ only triggered new bone formation while intervening the healing process at the phase of tissue inflammation (usually within 1-7 days after the surgery). However, delayed or prolonged treatment of Mg2+ intervention would compromise bone formation unexpectedly by up to 2.5 times. When the rat’s immune system (macrophages) was depleted by immuno-suppressor (liposome clodronate) via intraperitoneal injection, the bone-inducing effects of Mg2+ were completely abolished. Therefore, we believe that the bone-enhancing reaction of Mg2+ is likely to be mediated through the modulation of macrophages.
Our in vitro studies further demonstrated that Mg2+ has contributed to an upregulated expression of TRPM7, and a TRPM7-dependent influx of Mg2+ in the monocyte-macrophage lineage, resulting in the cleavage and nuclear accumulation of TRPM7-cleaved kinase fragments (M7CKs). This reaction then triggered the phosphorylation of Histone H3 at serine 10, in a TRPM7-dependent manner at the promoters of inflammatory cytokines, leading to the formation of a pro-osteogenic immune microenvironment. In the later bone remodelling phase, however, the continued exposure of Mg2+ not only led to the over-activation of NF-κB signalling in macrophages and increased number of osteoclastic-like cells, but also decelerated bone maturation through the suppression of hydroxyapatite precipitation. Thus, the negative effects of Mg2+ on bone regeneration could override the initial pro-osteogenic benefits of Mg2+.
Significance
The HKUMed research team is the first to systematically analyse the dose- and time-dependent effects of Mg2+ on the monocyte-macrophage-osteoblast axis in bone repair and unveils its underlying mechanisms. These research findings have enhanced the understanding of the role of Mg2+ in bone healing, paving the way for better utilisation of the functional effects of Mg2+ in bone regeneration and development of Mg2+ containing, degradable biomaterials for more effective treatment of bone fractures.
‘There is a very important message arising from this study. When we aim to achieve superior bone fracture healing or bone growth by using cationic minerals such as Mg2+, a precise protocol, especially “timing and right dosage”, must be taken into account. Otherwise, the treatment is just like “backfire”,’ said Professor Kelvin Yeung Wai-kwok of Department of Orthopaedics and Traumatology, HKUMed, who led the research. ‘With better understanding of the roles of Mg2+ in bone healing, we can then exert a paradigm shift in the clinical treatments of bone fracture and even osteoporosis treatment that may harness the healing power of Mg2+ and other cations.’
About the research team
This research was led by Professor Kelvin Yeung Wai-kwok of Department of Orthopaedics and Traumatology, HKUMed. The first author Dr Qiao Wei is post-doctoral fellow of Department of Orthopaedics and Traumatology, HKUMed. Professor Chen Zhuofan, Guanghua School of Stomatology, Sun Yat-sen University and Dr Lam Yun-wah, Associate Professor, Department of Chemistry, City University of Hong Kong are the co-corresponding authors. The research interests of Professor Yeung’s team include orthopaedic biomaterials, musculoskeletal tissue regeneration and anti-bacterial infection.
Acknowledgements
This study was supported by the National Key R&D Program of China (R&D#2018YFA0703100), General Research Fund of Hong Kong Research Grant Council (#17214516, #N_HKU725/16), Sanming Project of Medicine in Shenzhen “Team of Excellence in Spinal Deformities and Spinal Degeneration” (SZSM201612055), Shenzhen Science and Technology Funds (JSGG20180507183242702), Hong Kong Innovation Technology Fund (ITS/287/17 and ITS/405/18), the Science and Technology Commission of Shanghai Municipality (No. 18410760600), International Partnership Program of the Chinese Academy of Sciences (GJHZ1850), National Natural Science Foundation of China (81970975), and Guangdong Financial Fund for High-Caliber Hospital Construction (174-2018-XMZC-0001-03-2125/D-10).
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).
