Media
HKU and international collaborative research teams
identify a novel PARP-1 complex that promotes damaged DNA repair raising new possibility of target therapy for breast cancer and ovarian cancer
29 Sep 2015
PARP-1 is an enzyme with poly(ADP-ribosyl)ation activity, and has been demonstrated to play an important role in DNA damage response. Upon DNA damage, PARP-1 rapidly accumulates at sites of DNA break, and recruits many DNA repair proteins through poly(ADP-ribose) binding for efficient DNA repair. Since the 1980s, PARP-1 has been considered as a promising therapeutic target for anticancer therapy. A PARP inhibitor has recently been approved by Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the clinical treatment of ovarian cancers in women with BRCA mutations. One of the proposed mechanisms for PARP inhibitors is called synthetic lethality, by which PARP inhibitors would preferentially kill cancer cells with BRCA mutations but not normal cells. However, the exact mechanism of PARP inhibitors is not completely clear yet and this treatment still shows various side effects and drug resistance.
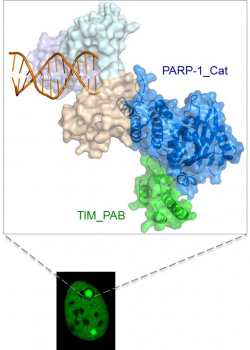
In a collaborative effort, researchers from The University of Hong Kong (HKU), Karolinska Institutet and Hong Kong University of Science and Technology find that disruption of PARP-1-Timeless interaction compromises homologous recombination (HR) repair, while PARP inhibitors cannot break the PARP-1-Timeless complex in cells. The team also identifies the novel PARP-1-Timeless complex that can promote damaged DNA repair. The findings of this study provide implications for understanding the mechanism of the clinically active PARP inhibitors and raise a new possibility to develop alternative PARP-1 target therapy for cancer treatment in the future studies. The study has been published online in Molecular Cell this month.
Significance of research
Dr Chengmin Qian, Assistant Professor of School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, HKU, and who coordinated this collaboration, pointed out that, “The study shows that disruption of the interaction of PARP-1 with Timeless compromises the efficient DNA double-strand break (DSB) repair. This may provide an alternative anticancer strategy to target PARP-1 by developing new drug to block the interaction of PARP-1 with Timeless, and the detailed structural information provided in this study can greatly facilitate this process.”
Research methods and findings
In this study, the authors reported a novel interaction between PARP-1 and Timeless that is independent of poly(ADP-ribosyl)ation. By using X-ray crystallography, the team also determined the crystal structure of the Timeless and PARP-1 complex. Taking advantage of live cell imaging and FRAP assay, the team further demonstrated that PARP-1 and Timeless complex is rapidly recruited to the DNA DSB sites to facilitate the HR repair. The recruitment of Timeless to DNA damage sites depends on the physical interaction with PARP-1, but is independent of PARP-1 enzymatic activity. The crystal structure explains why the interaction is independent of PARP-1 enzymatic activity, and why the clinically used PARP inhibitors cannot disrupt the interaction.
Previous studies
DNA stores genetic information that is replicated and passed from parental to daughter cells, so preservation of genome integrity and stability is essential for the health and viability of all organisms, including humans. However, cells are always under attack from multiple sources, such as UV light, environmental pollution, Reactive Oxygen Species (ROS), byproducts of cellular metabolism and cancer-causing factors from environmental sources. These factors can cause damages to DNA. Failure to respond to DNA damages may lead to irreversible chromosome fragmentation, developmental and neurological defects, premature aging and cancers. To combat DNA damages, cells evolve multiple DNA repair and checkpoints pathways to accurately and efficiently repair the damaged DNA. Both PARP-1 and Timeless have been demonstrated to play multiple roles in DNA damage response, but there is no report indicating that PARP-1 could function together with Timeless to preserve genome integrity and stability prior to this study.
About the research team
The collaborative study was conducted by Dr Chengmin Qian’s research group at School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, HKU, together with research teams led by Professor Thomas Hellday at Karolinska Institutet, Sweden and by Professor Randy Poon at Hong Kong University of Science and Technology (HKUST). Dr Qian’s group has interest in deciphering molecular mechanisms of DNA damage response, and his research is mainly funded by General Research Fund of The Research Grants Council. Both collaborators, Professor Thomas Helleday and Professor Randy Poon, are internationally renowned cancer biologists.
Please visit the website at http://www.med.hku.hk/tc/news/ for press photos.