Media
HKUMed reports that SARS-CoV-2 Omicron infection
recalls mRNA-vaccine-induced immunity for broad protection
27 Jan 2022
Researchers at AIDS Institute, Department of Microbiology, LKS Faculty of Medicine of The University of Hong Kong (HKUMed) and the State Key Laboratory of Emerging Infectious Diseases, HKU, have conducted a timely study for host immune responses in mRNA-vaccinees after acute infection by the alarming Omicron variant of SARS-CoV-2. The study demonstrated that mRNA-vaccinees had already displayed low levels of vaccine-induced neutralising antibodies and T cell responses at the time of infection. Upon vaccine breakthrough infection by the Omicron variant, mRNA-vaccinees mounted rapid broadly reactive neutralising antibodies and T cell responses, which contributed to the protection to the mRNA-vaccinees with mild clinical symptoms. The research paper is now online in the journal of Clinical and Translational Medicine [link to the publication].
Background
The world has entered an alarming wave of infection by the Omicron variant of SARS-CoV-2, which is more than 10 times the peak of the Delta variant in 2021. Due to a larger number of mutations were identified in the Omicron variant than that in the Delta variant, the biggest concern is whether current vaccine-induced memory immunity would confer any protection to the new viral variant. According to another study from HKUMed[1], vaccine-induced neutralising antibodies have shown significantly reduced ability to inhibit the Omicron variant in vitro, it is important to determine host immune responses among vaccinees after they became infected with the extremely transmissible Omicron variant.
Research methods and findings
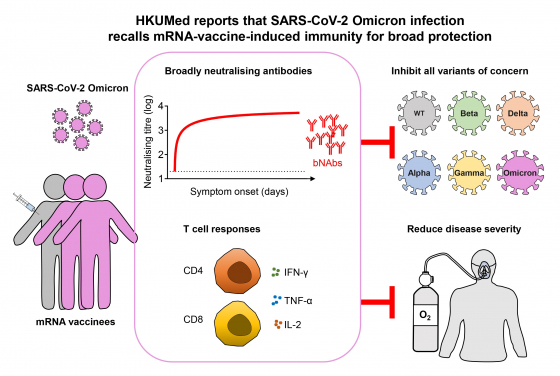
In this HKUMed study, two available local cases of Omicron infection were comprehensively and quantitatively studied for neutralising antibody amounts over time against all variants of concern including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P1), Delta (B.1.617.2) and Omicron (B.1.1.529) as compared with the wildtype (D614G). We consistently found that the Omicron variant showed the greatest resistance to BNT162b2 mRNA-vaccine‐induced neutralising antibodies as compared with other variants. These two mRNA-vaccinees with Omicron breakthrough infection, however, were able to elicit neutralising antibodies against the Omicron variant from unmeasurable levels to up to 75-fold higher than the mean peak amounts induced among vaccinees who received the 2-standard doses of BNT162b2. More importantly, the recalled neutralising antibodies displayed enhanced activities against all variants of concern tested.
In addition, the Omicron breakthrough infection also boosted antigen-specific T cell immune responses cross-reactive to the wildtype virus. The findings provided scientific evidence that mRNA-vaccine-induced memory immune responses were recalled quickly upon the Omicron breakthrough infection, which contributed to protection of the two cases.
Significance of the study
‘The findings suggested that receiving the complete mRNA-vaccinations is very important for protection against SARS-CoV-2 variants of concern,’ remarked Professor Chen Zhiwei, Director of AIDS Institute and Professor of the Department of Microbiology, HKUMed, who led the study, ‘Increasing rapidly the vaccine coverage among all age groups is urgently needed in Hong Kong before the wide spread of the Omicron variant. Our findings also implicate that the development of Omicron-targeted vaccines as booster is urgently needed to fight all current SARS-CoV-2 Variants of Concern (VOCs)’.
‘The use of one inactivated virus vaccine and one mRNA vaccine for COVID-19 prevention in HKSAR is unique’, said Professor Yuen Kwok-yung, Henry Fok Professor in Infectious Diseases and Chair of Infectious Diseases, Department of Microbiology, HKUMed; Director of the State Key Laboratory of Emerging Infectious Diseases, The University of Hong Kong, ‘Data on vaccinees post Omicron breakthrough infection is important to inform our vaccination policy in view of the rapidly waning of vaccine-induced immunity and emergence of new viral variants.’
About the research team
The research is led by Professor Chen Zhiwei, Director of AIDS Institute and Professor of the Department of Microbiology, HKUMed; and was conducted primarily by Dr Zhou Runhong, research officer; Dr Kelvin To Kai-wang, Head and Clinical Associate Professor, and Ms Peng Qiaoli, PhD candidate at the Department of Microbiology, HKUMed, shared the first authorship. This collaborative team also includes Dr Jacky Chan Man-chun, Dr Bosco Lam Hoi-shiu, Dr Owen Tsang Tak-yin from Princess Margaret Hospital; Dr Vivien Chuang Wai-man from Hospital Authority; Mr Cai Jianpiao, Assistant Research Officer, Department of Microbiology, HKUMed; Ms Liu Na, PhD candidate, AIDS Institute; Mr Huang Haode and Mr Yang Dawei and Mr Au Ka-kit, Research Assistants; and Professor Yuen Kwok-yung, Henry Fok Professor in Infectious Diseases and Chair of Infectious Diseases, Department of Microbiology, HKUMed; Academician, Chinese Academy of Engineering; Director of the State Key Laboratory of Emerging Infectious Diseases, The University of Hong Kong.
Acknowledgements
This study was supported by the Hong Kong Research Grants Council Collaborative Research Fund (C7156‐20GF, C1134‐20GF and C5110‐20GF) and Health and Medical Research Fund (19181012); Shenzhen Science and Technology Program (JSGG20200225151410198 and JCYJ20210324131610027); the Hong Kong Health@InnoHK, Innovation and Technology Commission; and the China National Program on Key Research Project (2020YFC0860600, 2020YFA0707500 and 2020YFA0707504); and donations from the Friends of Hope Education Fund. Professor Chen Zhiwei’s team was also partly supported by the Hong Kong Theme‐based Research Scheme (T11‐706/18‐N and T11-709/21-N).
About the Department of Microbiology, HKUMed
The academic staff of Department of Microbiology are actively involved in clinical service and basic research. Postgraduate students may pursue studies on various aspects of microbiology and infectious diseases leading to an MPhil or PhD degree. The Master of Medical Sciences programme offers an opportunity to postgraduates interested in more in-depth studies on the biomedical aspects of clinical microbiology and infectious disease. In addition, the clinical staff of the Department also participate in the training of clinical microbiologists in Hong Kong and Shenzhen. The Infectious Disease Courses and Postgraduate Diploma Programme provides a unique avenue for the training of qualified medical practitioners in infectious diseases.
To promote knowledge exchange, research activities at the Department of Microbiology, HKUMed can be viewed through http://www.microbiology.hku.hk/.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).
[1] https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab1041/6463504?login=false