Media
Four HKU Research Projects Awarded US National Academy of Medicine Healthy Longevity Catalyst Award (Hong Kong) 2023
15 Oct 2023
Four research projects from the Faculty of Engineering and the Li Ka Shing Faculty of Medicine at the University of Hong Kong (HKU) were awarded the Healthy Longevity Catalyst Awards (Hong Kong) in the Healthy Longevity Global Competition held by the US National Academy of Medicine.
The Healthy Longevity Global Competition is a series of awards and prizes that aim to catalyse breakthrough innovations to improve people’s physical, mental, and social health and well-being as they age. It includes three phases – the Catalyst Phase, the Accelerator Phase, and the Grand Prize Phase.
In 2022, the Research Grants Council (RGC) under the University Grants Committee of Hong Kong began collaborating with NAM to host the Healthy Longevity Catalyst Innovation Award (Hong Kong). As part of the Catalyst Phase of the Competition, the award aims to support bold, innovative ideas in any academic field that could potentially extend human healthspan, including research projects aimed at improving the health status of individuals across all age groups.
The Healthy Longevity Catalyst Awards (Hong Kong) presents ten awards each year. Each winner receives up to $50,000 USD (approximately HKD$ 389,000) in research funding for one year and becomes eligible for the Accelerator Phase and subsequent phrase of the program. Additionally, winners are invited to attend the Innovation Summit hosted by NAM, where they can share their research achievements with other awardees, policymakers, and investors. The RGC also provides a maximum allowance of 180,000 Hong Kong dollars to the winning research teams to facilitate their attendance at the Innovation Summit.
The four HKU award-winning projects are:
Project One
Reverse inflammaging by targeting nuclear PD-L1 in inflammatory macrophages
Principal Investigator: Dr Rio Sugimura, Assistant Professor, School of Biomedical Sciences, LKS Faculty of Medicine
About the project:
Inflammaging is one of the major drivers of aging and senescence. The identification of its mechanism will enable drug discovery to stop aging. Inflammatory macrophage is the main contributor to inflammaging. Dr Sugimura's research team recently identified that nuclear PD-L1 regulates inflammatory programs in macrophages. Loss of nuclear PD-L1 inhibits the production of inflammatory cytokines of macrophages, suggesting a promising target for anti-aging therapeutics. The team proposes to determine the molecular mechanisms of nuclear PD-L1 and discover a therapeutic approach to inhibit inflammaging. The feasibility of the project is demonstrated by preliminary data and accumulated literature. The success of the project will lead to drug discovery and R&D application in the industry of Hong Kong. The impact of targeting inflammaging for human health is immense.
Project Two
Leveraging brown adipose tissue to combat elderly stroke
Principal Investigator: Dr Leiluo Geng, Research Assistant Professor, Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine
About the project:
Brown adipose tissue (BAT), consisted of brown adipocytes and stromal vascular fraction, plays a pivotal role in regulating thermogenesis and energy expenditure. BAT also modulates systemic metabolism and health via secreting endocrine factors, termed batokines. Ageing is associated with loss and dysfunction of BAT and poor prognosis of stroke, which has been a leading cause of death and disability in the aged population. Whether restoration of BAT function is able to alleviate stoke injury in the elderly remains unknown. Preliminary studies by the research team demonstrated that both surgical and genetic depletion of BAT exacerbated ischemic stroke injury induced by middle cerebral artery occlusion in young mice. Aged mice transplanted with BAT from young mice showed alleviated cerebral injury after stroke, suggesting the protective role of functional BAT in elderly stroke. Additionally, BAT-derived exosomes, which contain multiple kinds of batokines, were found to be significantly alleviated the cerebral ischemia injury in aged mice. Based on these findings, the study aims to comprehensively reveal the beneficial role of BAT in modulating elderly stroke injury, clarify the fine-tuned crosstalk between BAT and central nervous system in stroke injury, and identify novel batokines of therapeutic potential for elderly stroke. It will provide phonotypic and mechanistic insights into both stroke biology and brown adipocyte biology as well as contribute to the development of BAT-based therapies for elderly stroke.
Project Three
An Integrated Graph Convolutional Network Multimodal Platform (GCN-MP) for Early Detection and Prediction of Late Onset Alzheimer’s Disease
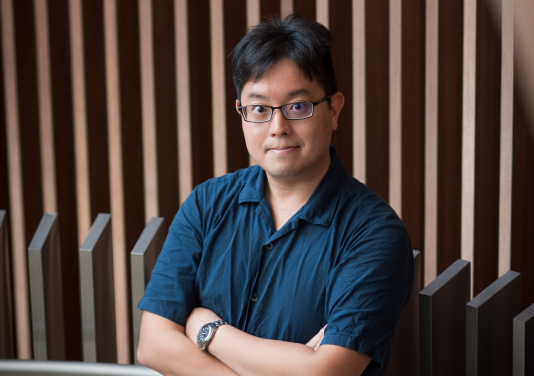
Principal Investigator: Professor Victor O.K. Li, Chair Professor of Information Engineering, Department of Electrical and Electronic Engineering, Faculty of Engineering
About the project:
Alzheimer’s disease (AD) is a leading cause of death worldwide. Early prediction of Late Onset Alzheimer’s disease (LOAD) is critical to timely intervention before irreversible brain damage. Accurate early LOAD prediction is challenging due to the need for a wide range of multimodal assessments, which can be time-consuming, costly, and invasive, preventing those who might develop symptomatic LOAD from timely diagnosis and therapeutic interventions. An AI-driven-multimodal LOAD prediction based on readily available data as inputs to an AI model trained on heterogenous data of high-dimensional modalities/features across different diseases/populations, can be desirable for early LOAD prediction/intervention. The transformative multimodal platform aims, FIRST, to develop an integrated Graph Convolutional Network Multimodal Platform (GCN-MP) technology, to fuse small datasets of different modalities/features/diseases/populations via a multimodal GCN model for accurate prediction of LOAD onset age; SECOND, to uncover early biomarkers and principal pathways driving LOAD onset. Five novelties have been proposed, including, heterogeneous multimodal inputs, multimodal data fusion, potential causal pathways identification, and integrated multimodal risk scores (IMRS) based on high-saliency complementary modalities. The work will greatly facilitate clinicians and at-risk AD populations to more conveniently and accurately predict LOAD onset via the IMRS and onset age prediction, based on their available data types, and advance our understanding of LOAD etiology. The research team’s two consecutive grants awarded by NAM, and the numerous works done on AI-driven prediction and estimation and in neurological studies from our members in HKU-Cambridge-AI-for-Neuro-disease-Research-Platform have prepared us to achieve these meaningful goals of AI for Social Good.
Project Four
Enabling the effective and safe epigenetic rejuvenation with re-engineered transcription factors
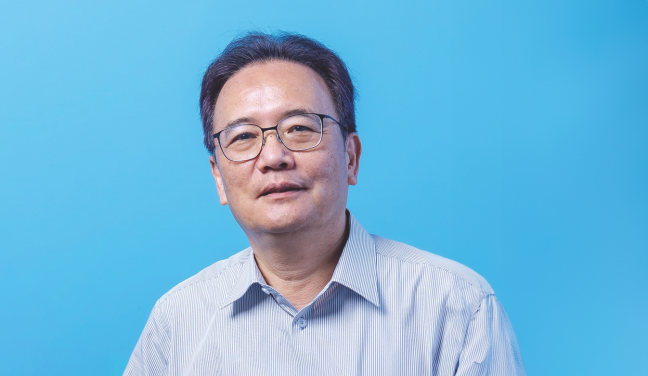
Principal Investigator: Dr Ralf Jauch, Associate Professor, School of Biomedical Sciences, LKS Faculty of Medicine
About the project:
The Yamanaka factors Oct4, Sox2, Klf4 and c-Myc can convert somatic tissues into induced pluripotent stem cells resembling the inner cell mass of pre-implantation embryos. Induction of pluripotency is accompanied by a remarkable rejuvenation of cells from aged donors so that they epigenetically, structurally and metabolically resemble newborn tissue. The time restricted expression of all or some of these factors can repair damaged tissues in mice. However, the prolonged expression of Yamanaka factor induces tumors preventing the application of this strategy for human gene therapy. Dr Jauch's research team has identified several re-engineered transcription factors (eTFs) that enhance pluripotency and direct lineage reprogramming. They hypothesize that replacing the Yamanaka factors with eTFs can decouple epigenetic rejuvenation from pluripotency induction, speeds up age reversal, enhances safety and relieves cargo restrictions for gene delivery. The reseachers have previously identified re-engineered SOX17 (eSOX17), a miniaturized SOX (miniSOX) as well as enhanced POU and KLF factors (ePOU and eKLF) with established activity to convert mouse and human cells into pluripotent or multipotent stem cells. They will overexpress combinations of these eTFs in aged human fibroblasts and measure the epigenetic and cellular rejuvenation. The study aims to identify factor combinations that lose the full potential to induce pluripotency but retain the ability to rejuvenate cells akin the full complement of Yamanaka factors. The unique eTF centered approach could lead to the identification of a strategy that not only makes rejuvenation more efficacious and delivery easier but also eliminates the imminent risk of oncogenesis associated with classical Yamanaka factors.
Research Grants Council: https://www.ugc.edu.hk/big5/rgc/funding_opport/hlca/
The US NAM Healthy Longevity Challenge: https://healthylongevitychallenge.org/about-us/
Media enquiries
Communication and Public Affairs Office
Ms Melanie Wan (Tel: 2859 2600 / Email: melwkwan@hku.hk)
Ms Jaymee Ng (Tel: 3910 3612 / Email: ngjaymee@hku.hk)
Mr Kenneth Choi (Tel: 2859 2607 / Email: khkchoi@hku.hk)